Home >> News center
-
 201907-01
201907-01Zuo Wei was elected China's top ten innovators in 2018
Peoples Pictorial (Overseas Edition), a well-known publication, recently selected 10 innovative figures in 2018. Professor Zuo Wei was elected successfully.
-
 201907-01
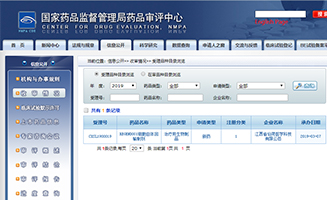
201907-01REGEND001 New Drug Clinically Accepted by CFDA
The application for clinical trial of REGEND001 cell autotransfusion preparation, a product independently developed by the company, has been accepted by the Drug Review Center of the State Drug Administration with the acceptance number CXSL1900019.
-
 201907-01
201907-01The company's project was selected as the top ten progress of 2018 biotechnology
On January 12, 2019, the announcement of "2018 China Medical Biotechnology Top Ten Progress Awards " co-sponsored by China Medical Biotechnology Association and China Pharmaceutical Biotechnology Magazine was held in Tonglu, Zhejiang Provinc…